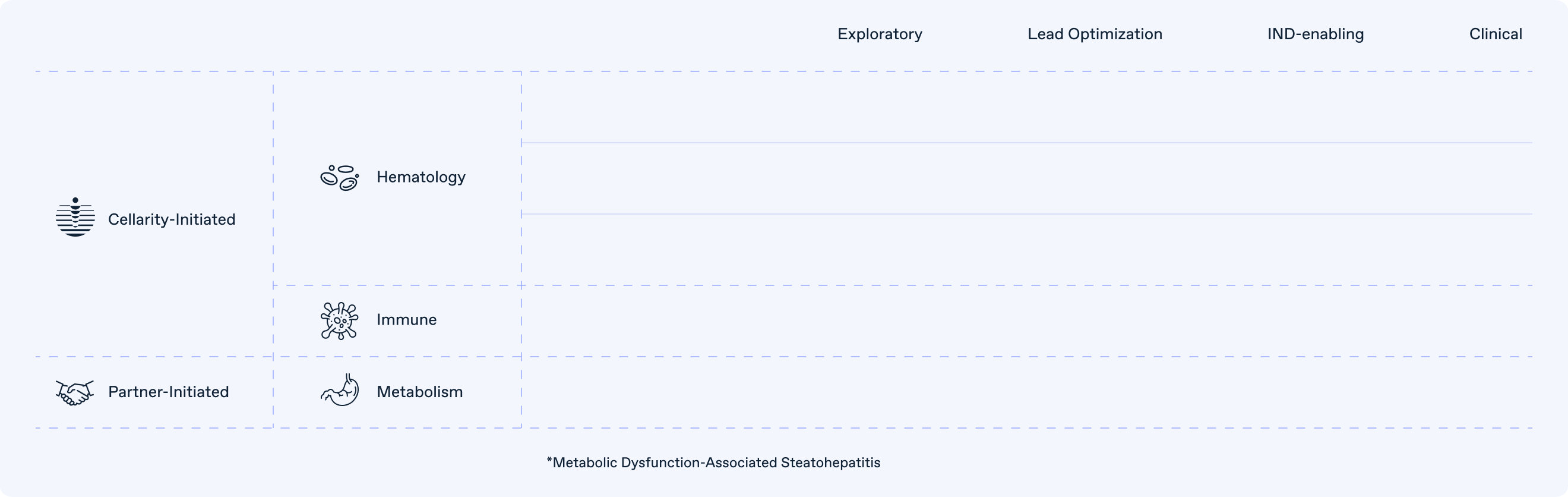
Pipeline
Cellarity is creating “game-changing” drugs that are out of reach today.
Having proven the concept of our platform across diverse cell types and cell behaviors, we have selected hematology and immunology as our first therapeutic areas (TA) to build out a diverse portfolio of Cellarity-initiated programs that leverage the strong scientific capabilities that we have developed.
To fulfill and accelerate the broad potential of our platform, we have partnered programs applying our disruptive approach to areas of strategic interest to partner companies. Our first example of this is our program addressing novel biologic insights in Metabolic Dysfunction-Associated Steatohepatitis (MASH) in partnership with Novo Nordisk.
Scroll sideways to view more
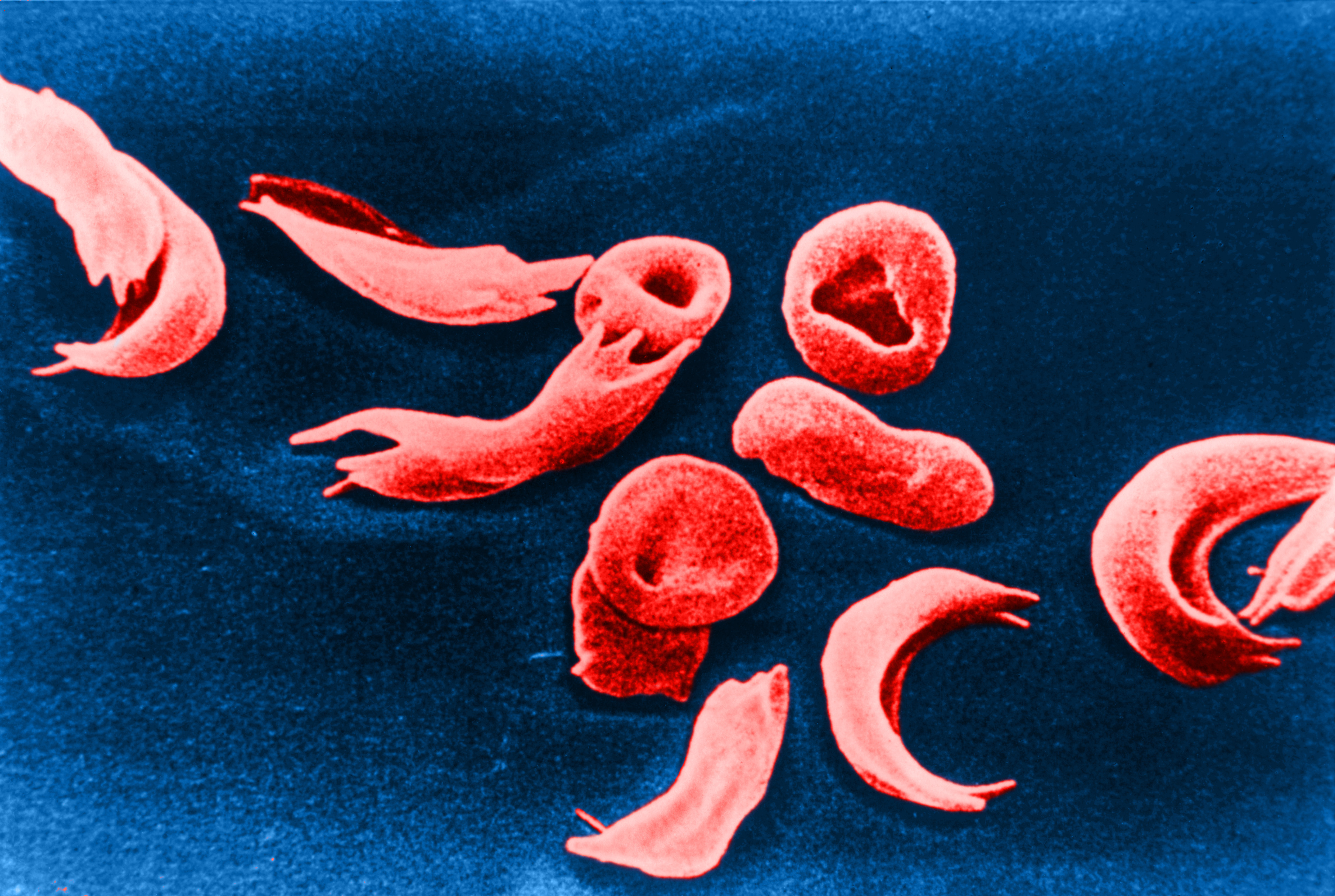
CLY-124: A First-in-Class Globin-Switching Oral Medicine for the Treatment of Sickle Cell Disease
CLY-124 is designed to be a first-in-class, oral therapeutic to treat sickle cell disease through globin switching. This mechanism aims to increase fetal hemoglobin to replace and dilute sickle hemoglobin, resulting in reduction of vaso-occlusive crises. Sickle cell disease is an inherited, devastating disease involving red blood cells that change shape to a sickle, crescent shape that blocks small blood vessels. The disease affects millions of people worldwide, causing extreme pain crises, fatigue, multi-organ damage, chronic and progressive inflammation, and shortened lifespan.
About CLY-124:
CLY-124